EU to Relax GMO Rules as Part of New Vaccine Strategy
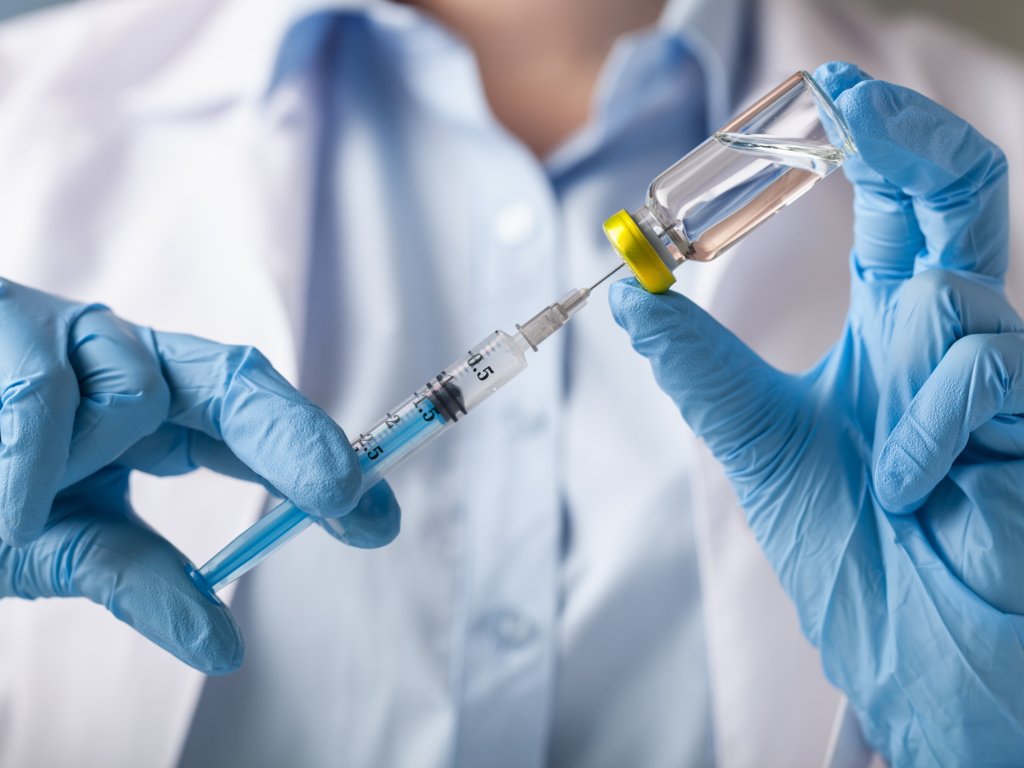
Brussels is ready to loosen its stance on GMOs in order to avoid bottlenecks in clinical trials for a coronavirus vaccine involving multiple countries, Euractiv reports.
Current GMO legislation does not foresee situations of urgency, resulting in very complex and time-consuming procedures, the Commission argues, saying “there is considerable variety across member states in the national requirements and procedures implementing the GMO directives.”
The proposed derogation, which still needs to be voted by the European Parliament and the EU Council of Ministers, will last for as long as COVID-19 is regarded as a public health emergency, the Commission said.
The relaxed rules will apply not only for clinical trials on a COVID-19 vaccine but also for treatments, the Commission’s communication reads, although compliance with good manufacturing practices (GMPs) and an environmental risk assessment of the products will still be carried out before their marketing authorization.
The Commission called on the EU’s co-legislators to adopt the proposal swiftly in order to enable clinical trials to take place in Europe as quickly as possible. German MEP Peter Liese, European People’s Party (EPP) spokesperson for health, agreed with the Commission’s push on passing the derogation as soon as possible, saying that it needs to be done before the summer break.
– It’s a challenge to do it in 3-weeks time, but at the Parliament, we have followed fast-track procedures for less crucial issues in the past – he said.
Although GMOs are controversial, Liese said the proposed temporary change in legislation would be “small” and “very targeted”, Euractiv adds.
Most Important News
06.04.2024. | Agriculture
Preconditions for Placement of Fresh Blueberries and Dried Plums in Chinese Market Secured

16.04.2024. | News
Jovan Ciric, Leasing Director Retail MPC Properties – MPC Echo symbolizes our desire for good ideas and innovative endeavors to spread freely and bring about positive changes

16.04.2024. | News
10.04.2024. | Finance, IT, Telecommunications, Tourism, Sports, Culture
Creative Industry – What This Serbian Economy Sector Worth EUR 2 Billion Encompasses

10.04.2024. | Finance, IT, Telecommunications, Tourism, Sports, Culture
18.04.2024. | Industry, Finance
Here come the new hunters for Serbian gold – Australian Strickland Metals buys mining project on mountain Rogozna

18.04.2024. | Industry, Finance
16.04.2024. | News
Economy Fair in Mostar opens – 26 companies from Serbia exhibiting

16.04.2024. | News
18.04.2024. | Transport
Jovanovic: Purchase of Siemens trams produced in Kragujevac for GSP Beograd should be considered

18.04.2024. | Transport


 Izdanje Srbija
Izdanje Srbija Serbische Ausgabe
Serbische Ausgabe Izdanje BiH
Izdanje BiH Izdanje Crna Gora
Izdanje Crna Gora


 News
News







