Torlak gets permit to produce vaccine against flu
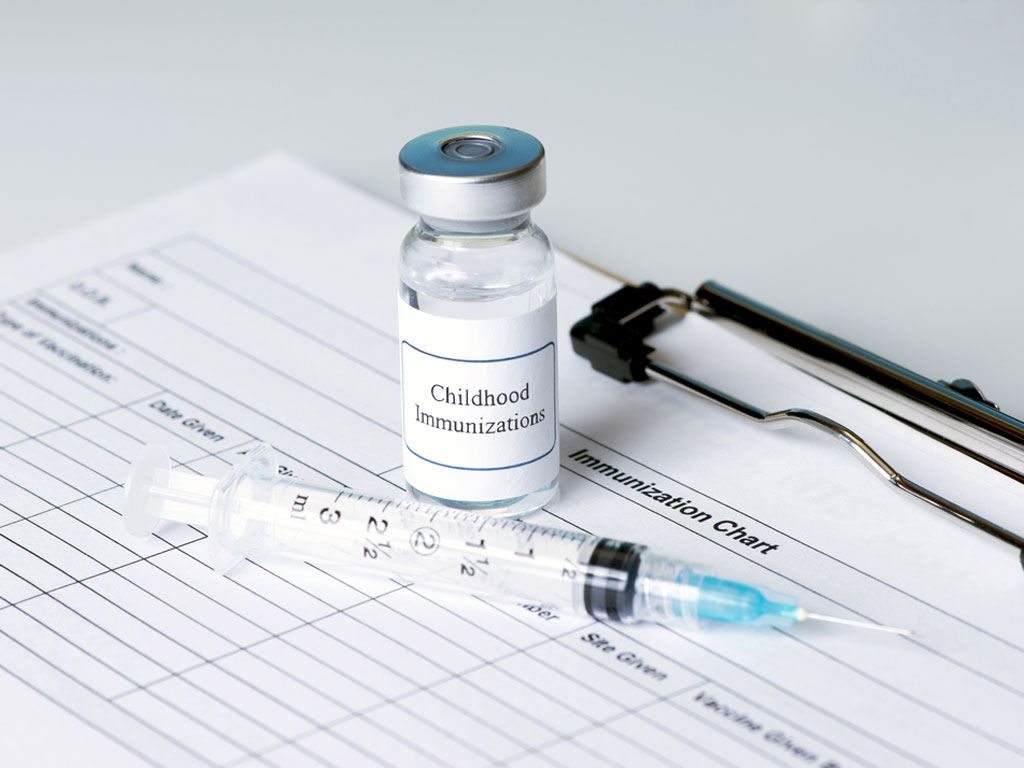
Torlak has got the Certificate of Compliance of Drug Manufacturing with the Guidelines of Good Manufacturing Practices for the medicine Vaccine Against Flu, splitted, inactivated, intended for clinical trials.
The release reads that this certificate is of exceptional importance for Torlak which expects the vaccine to successfully pass all clinical trials and then be made available to citizens for the purpose of preventing seasonal flu.
Most Important News
06.04.2024. | Agriculture
Preconditions for Placement of Fresh Blueberries and Dried Plums in Chinese Market Secured

16.04.2024. | News
Jovan Ciric, Leasing Director Retail MPC Properties – MPC Echo symbolizes our desire for good ideas and innovative endeavors to spread freely and bring about positive changes

16.04.2024. | News
10.04.2024. | Finance, IT, Telecommunications, Tourism, Sports, Culture
Creative Industry – What This Serbian Economy Sector Worth EUR 2 Billion Encompasses

10.04.2024. | Finance, IT, Telecommunications, Tourism, Sports, Culture
23.04.2024. | Finance
Mali: UK Export Finance interested in financing several projects in Serbia

23.04.2024. | Finance
16.04.2024. | News
Economy Fair in Mostar opens – 26 companies from Serbia exhibiting

16.04.2024. | News
23.04.2024. | Construction, Transport
Tender for first section of Belgrade-Nis fast railroad from Velika Plana to Paracin announced

23.04.2024. | Construction, Transport


 Izdanje Srbija
Izdanje Srbija Serbische Ausgabe
Serbische Ausgabe Izdanje BiH
Izdanje BiH Izdanje Crna Gora
Izdanje Crna Gora


 News
News










